38 fda health claims on food labels
Label Claims for Food & Dietary Supplements | FDA Mar 07, 2022 · Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims ... How to Start a Food Business | FDA - U.S. Food and Drug ... Food Businesses Subject to FDA Regulation. FDA regulates all foods and food ingredients introduced into or offered for sale in interstate commerce, with the exception of meat, poultry, and certain ...
Label Claims for Conventional Foods and Dietary Supplements Mar 07, 2022 · Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims ...
Fda health claims on food labels
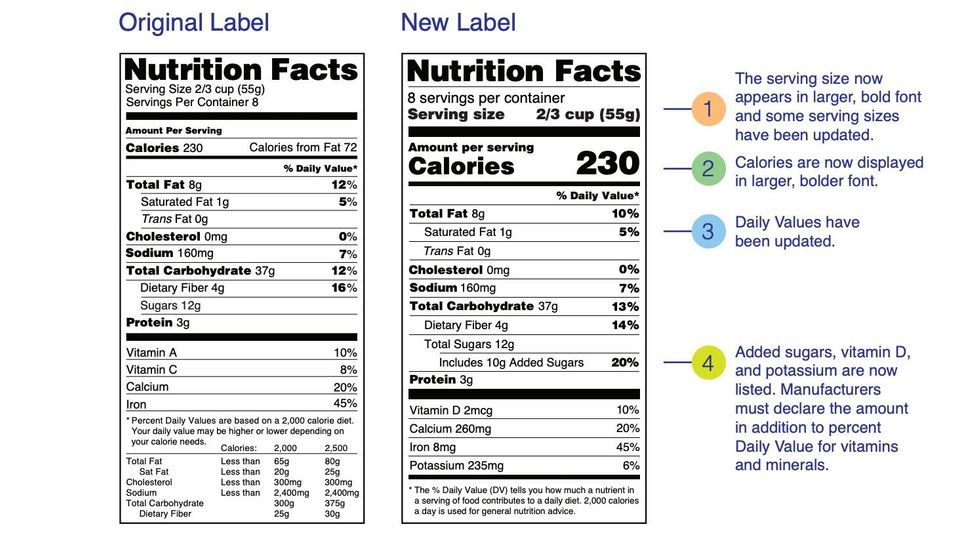
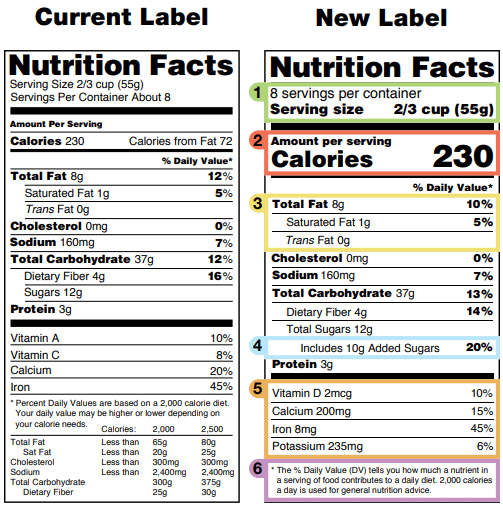
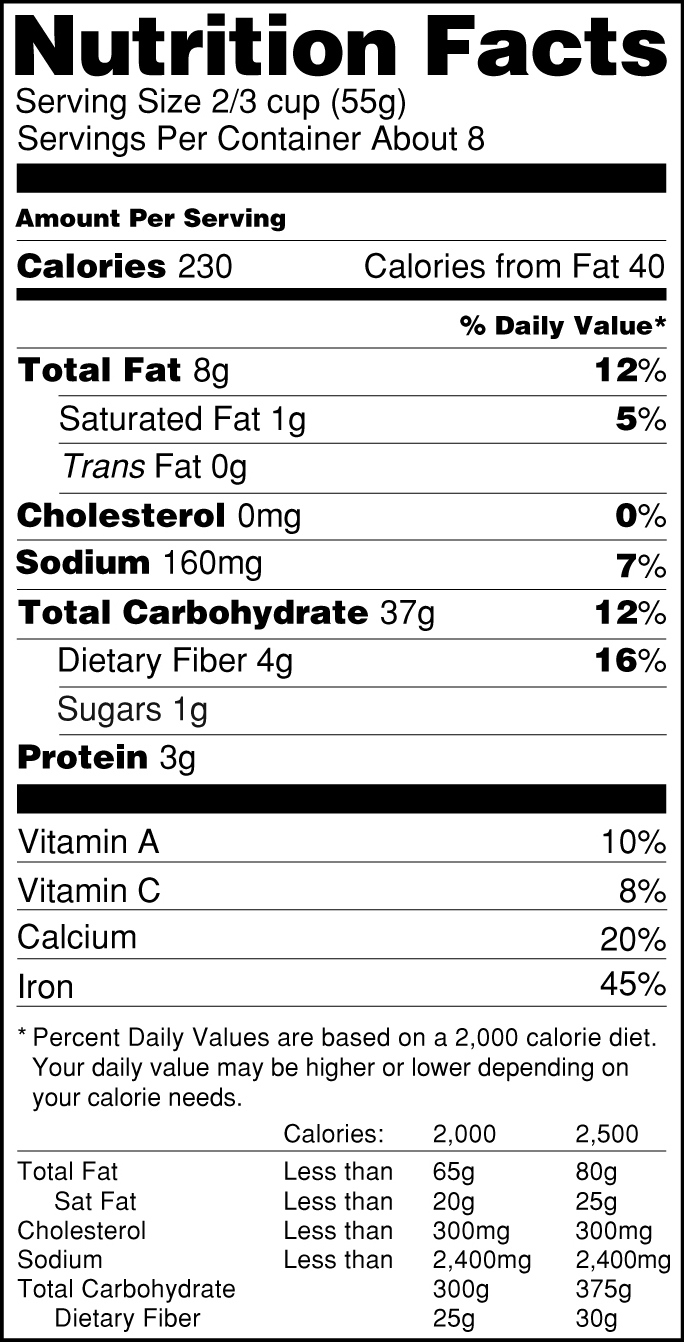
Food/Dietary Supplement Guidance and Regulatory Information May 16, 2022 · 2014. WITHDRAWN Acidified and Low-Acid Canned Foods: (DRAFT) Submitting Form FDA 2541 (Food Canning Establishment Registration) and Forms FDA 2541d, FDA 2541e, FDA 2541f, and FDA 2541g (Food ... Industry Resources on the Changes to the Nutrition Facts Label Sugar content claims described in 21 CFR 101.60(c), such as “sugar free” and “no sugar,” are required to be accompanied by a statement that the food is “not a reduced calorie food ... Health News | Latest Medical, Nutrition, Fitness News - ABC ... Sep 22, 2022 · The new chief at the Food and Drug Administration's tobacco center has inherited a raft of problems September 26 Pfizer submits bivalent COVID booster to FDA for use in children ages 5 and up
Fda health claims on food labels. Organic on Food Labels | FDA Mar 07, 2022 · For more information on the use of the term “organic” on food labels and USDA requirements, go to the National Organic Program website. Content current as of: 03/07/2022 Health News | Latest Medical, Nutrition, Fitness News - ABC ... Sep 22, 2022 · The new chief at the Food and Drug Administration's tobacco center has inherited a raft of problems September 26 Pfizer submits bivalent COVID booster to FDA for use in children ages 5 and up Industry Resources on the Changes to the Nutrition Facts Label Sugar content claims described in 21 CFR 101.60(c), such as “sugar free” and “no sugar,” are required to be accompanied by a statement that the food is “not a reduced calorie food ... Food/Dietary Supplement Guidance and Regulatory Information May 16, 2022 · 2014. WITHDRAWN Acidified and Low-Acid Canned Foods: (DRAFT) Submitting Form FDA 2541 (Food Canning Establishment Registration) and Forms FDA 2541d, FDA 2541e, FDA 2541f, and FDA 2541g (Food ...




























:no_upscale()/cdn.vox-cdn.com/uploads/chorus_asset/file/3652082/healthy-choice.0.png)
Post a Comment for "38 fda health claims on food labels"